
- #CHEMICAL EQUATION BALANCER WITH STEPS HOW TO#
- #CHEMICAL EQUATION BALANCER WITH STEPS PLUS#
- #CHEMICAL EQUATION BALANCER WITH STEPS FREE#
Each unique substance in the chemical reaction is sunder by a plus sign (+). Click here to get an answer to your question what are the steps involved in balancing a chemical equation khushi556 khushi556 Chemistry Secondary School. The first step to balance the equation is to write down the chemical formula of reactants that are listed on the left side of the chemical equation. Step 1: Write Down the Unbalanced Equation. The reactants and products are sunders by arrow symbols. If you also find difficulty in balancing the chemical equations, follow the steps below. The input equation should be in the following formatĪ chemical equation is interpreted as the symbolic representation of the chemical reaction where the reactants are written on the left side and the products are written on the right side. Method to use the Ionic net equation calculator is as follows:ġ: Enter the chemical equation in the “Enter the chemical equation” field.Ģ: Now click the button “Balance” to get the equalize equation.ģ: Finally, for the specified chemical equation, a window will pop with the output.

#CHEMICAL EQUATION BALANCER WITH STEPS HOW TO#
How to use Net Ionic Equation Calculator? The balanced net ionic equation calculator tool makes the prediction quick and easier and displays the answer in a fraction of seconds.
#CHEMICAL EQUATION BALANCER WITH STEPS FREE#
In this situation, the number of atoms on each side can be tabulated as follows.Net ionic and ionic equation calculator is a free online tool that shows the structure, equilibrium constant, balanced equation, substance properties with chemical formulas and names. Also, it describes the simple steps to balance chemical equation, as well as the Balancing Equation definition. Step 2: On the reactant and product sides, compare the total number of atoms of each element. Balancing Equations Calculator: Free Balancing Equations Tool is a wonderful tool that helps you in balancing any type of chemical equation. It creates carbon dioxide (CO2) and water when it comes into touch with oxygen (O2) (H2O). The molecular formula for propane is C3H8. How To Balance Chemical Equations In 5 Easy Steps Balancing Tutorial You. Chemistry Equation Balancing Calculator By Chrysalis Innovations. Easy Steps For Balancing Chemical Equations. Step 1: Create an unbalanced equation using the chemical formulae of the reactants and products (if it is not already provided). 4 Best Free Balancing Chemical Equations Calculator For Windows. The combustion process between propane and oxygen is used as an example to demonstrate this method. Ethane, C 2 H 6, reacts with oxygen to make carbon dioxide and water.

When it comes to balancing chemical equations, the first step is to acquire the entire imbalanced equation. Write and balance the chemical equation for each given chemical reaction. Now we have the same exact number of iron and oxygen on the left-hand side as on the right-hand side. So, on the left-hand side apply two atoms of oxygen with one and a half molecules. Stoichiometric coefficients are assigned in a way that balances the total number of atoms of an element on the reactant and product sides when balancing chemical equations. We need to make it equal but chemical equation product calculator automatically equals it.
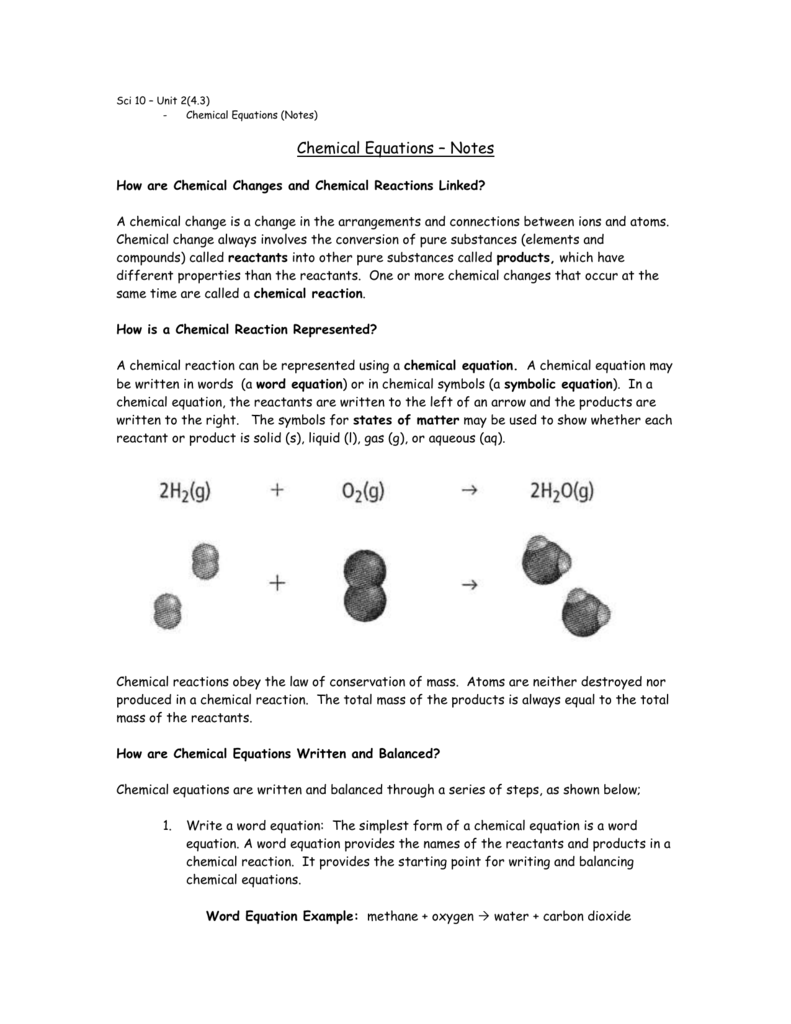
The stoichiometric coefficients of O2 and H2O in the reaction CH4 + 2O2 → CO2 + 2H2O are 2 and 1, respectively, in the equation CH4 + 2O2 → CO2 + 2H2O. It calculates the ratio between the responding species and the reaction products. The total number of molecules of a chemical species that participate in a chemical reaction is described by a stoichiometric coefficient. The part of the chemical equation to the left of the ‘→' sign is the reactant side, while the part to the right of the arrow symbol is the product side. This is crucial because a chemical equation must follow the laws of conservation of mass and constant proportions, which means that the reactant and product sides of the equation must have the same amount of atoms of each element.Ī chemical equation is a symbol for a chemical reaction in which the reactants and products are represented by their chemical formulae.ĢH2 + O2 →2H2O is an example of a chemical equation that depicts the reaction between hydrogen and oxygen to generate water. The inclusion of stoichiometric coefficients to the reactants and products is required to balance chemical equations.


 0 kommentar(er)
0 kommentar(er)
